Vulcanization
| A service provided by |
|---|

|
| Polymer Service GmbH Merseburg |
| Tel.: +49 3461 30889-50 E-Mail: info@psm-merseburg.de Web: https://www.psm-merseburg.de |
| Our further education offers: https://www.psm-merseburg.de/weiterbildung |
| PSM on Wikipedia: https://de.wikipedia.org/wiki/Polymer Service Merseburg |
Vulcanization process
General basics
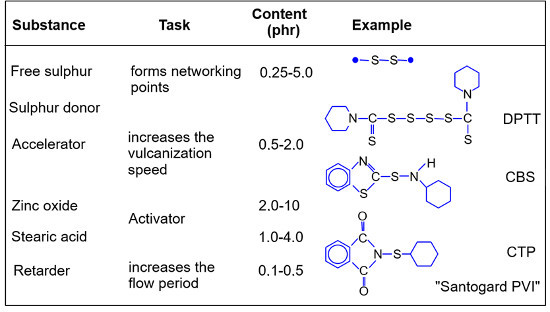
Rubber compounds can be made up of a variety of components. In addition to the rubber, filler, processing aid, plasticizer and other additives, a cross-linking system is mixed in, depending on the polymer. For example, sulphur cross-linking is the preferred method for cross-linking diene rubbers (NBR, SBR, NR or BR). In addition to accelerator and activator (see Table 1), elemental sulphur, which is present in the form of S8-rings, is often used for this purpose. The amount of sulphur required depends on the amount of vulcanization accelerator and the required vulcanizate properties. The use of sulphur donors releases sulphur during vulcanization. Some sulphur donors are also vulcanisation accelerators and are then also dosed in larger quantities. This combination results in synergistic effects, through which the potential cross-linking possibilities of the sulphur donors are fully utilised. In order for the vulcanizate to achieve the properties relevant to the application, an accelerator must be mixed in alongside the sulphur. Almost all accelerators are only fully effective in the presence of metal oxides, of which zinc oxide (ZnO) has proven to be the best additive. The rubber/sulphur/accelerator/zinc oxide system is additionally activated by the addition of stearic acid or zinc stearate. This increases the solubility of the cross-linking system in the rubber by forming soluble complexes. Vulcanization retarders are used when vulcanization times are too short or high processing temperatures are required. This ensures sufficient processing safety [1].
| Table 1: Possible structure of a networking system [1] |
Models for the vulcanization process
There are several theories in the literature as to how vulcanization takes place chemically. The idea of Morrison and Porter [2] is listed in the literature as the most probable. According to this theory, an active accelerator complex is formed during cross-linking, which determines the duration of the incubation time. It is also certain that in the presence of zinc ions, complexes are formed that are soluble in the rubber. The active accelerator complex reacts with the sulphur and forms a sulphur transfer complex. The sulphur is then transferred to the rubber and finally cross-linking takes place. In addition to this simple reaction process, a series of subsequent and parallel reactions take place, which have different activation energies [3].
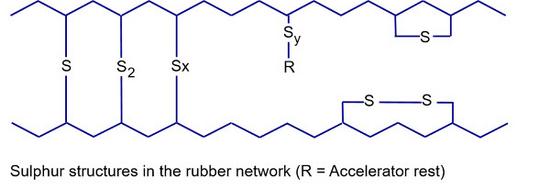
The incorporation of sulphur into the network during vulcanization can take place as a monosulphidic, disulphidic, polysulphidic, pendant sulphidic or cyclic monosulphidic and disulphidic grouping, which can be incorporated into the polymer matrix with the formation of pendant groups (SyR) (see Fig. 1). In addition to polysulphide degradation in the cross-linking sites, the subsequent reaction of cross-linking is the formation of cyclic thioethers on the rubber chain and zinc sulphide formation (from ZnO and polysulphidic sulphur). The mono- and disulphide structures result in lower permanent deformation, better thermal durability and lower reversion behaviour. In contrast, polysulphide cross-links can migrate along the chain at elevated temperatures, whereby there is no chain scission (slipping effect) and local stress peaks are reduced. The sulphur attacks the double bond in the polymer chain in the a-position (-C=C-C*-, *S-attack in the ally position). The cross-linking sites are approx. 30 to 100 monomer units apart [4].
| Fig. 1: | Sulphur structures in the rubber network [4] |
Determination of the vulcanization process with the aid of vulcametry
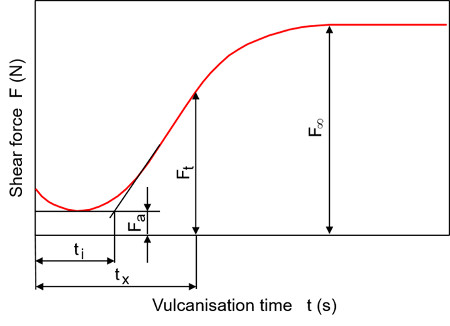
In practice, vulcametry is often used to determine the course of the cross-linking reaction, which is based on the proportionality between the shear modulus and the cross-linking density (see also: entropy elasticity). Vulcameters consist of a temperature-controlled reaction chamber and a force or torque measuring device. According to DIN 53529-1, the test can be carried out with different types of equipment [5]. When testing with a torsional shear vulcameter without a rotor, the specimen is subjected to an oscillating (sinusoidal) deformation of the specimen chamber. The periodic change in shear force or torque is recorded as a function of time. The course of the cross-linking reaction is shown schematically in Figure 2. The minimum value (Fa) of the cross-linking isotherms corresponds to the deformation resistance of the uncross-linked specimen.
| Fig. 2: | Cross-linking isotherm of a rubber compound according to DIN 53529-2 [6] |
In the majority of cross-linking systems, the cross-linking reaction does not start immediately, but with a time delay. This period is known as the incubation time (ti) and depends on the heating time of the sample as well as the upstream chemical reaction. In the case of sulphur cross-linking, for example, the incubation time is based on the delayed formation of the active accelerator complex.
Once the cross-linking reaction is complete, the vulcameter curve reaches a final value (F∞), which corresponds to the crosslinking density. The conversion of the cross-linking reaction in a rubber compound can be calculated by determining the conversion variable x, after the conversion time tx, according to equation (1).
| (1) |
See also
- Goodyear, Charles Nelson
- Degree of Cross-Linking Elastomers
- Rebound resilience elastomers
- Instrumented tensile impact test (ITIT), examples
- Dielectric properties
- Curing
References
| [1] | Elsner, P., Eyerer, P., Hirth, T. (Eds.): Kunststoffe Eigenschaften und Anwendungen. 8th revised and extended Edition, Springer Heidelberg (2012) (ISBN 978-3-642-16172-8; see AMK-Library under G 41) |
| [2] | Morrison, N. J., Porter, M.: Temperature Effects on the Stability of Intermedicates and Crosslinks in Sulfur Vulcanization. Rubber Chem. Technol. 57 (1984) 63–85; https://doi.org/10.5254/1.3536002 |
| [3] | Röthemeyer, F., Sommer, F.: Kautschuk Technologie. 2nd revised Edition, Carl Hanser Munich Vienna (2006) (ISBN 978-3-446-40480-9) |
| [4] | Schnetger, J.: Lexikon Kautschuktechnik. 3rd Edition, Hüthig Heidelberg (2004) (ISBN 978-3-7785-3022-1; see AMK-Library under K 7) |
| [5] | DIN 53529-1 (1983): Testing of Rubber and Elastomers – Measurement of Vulcanization Characteristics (Curometry) – General Working Principles |
| [6] | DIN 53529-2 (1983): Testing of Rubber and Elastomers – Measurement of Vulcanization Characteristics (Curometry) – Evaluation of Cross-linking Isotherms in Terms of Reaction Kinetics |